AlphaNine SD offers patients with hemophilia B an effective replacement therapy option
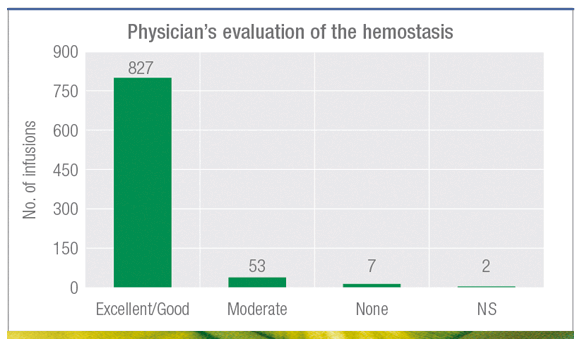
93% of infusions rated as excellent/good1
Pharmacokinetic, efficacy and safety study in 25 patients with severe hemophilia B treated with AlphaNine SD (1.6M IU used in 889 infusions).1
The 7 reported cases of no efficacy were explained by an insufficient initial dose compared to what was recommended by the study protocol. Six of seven bleedings were resolved with additional infusions.1
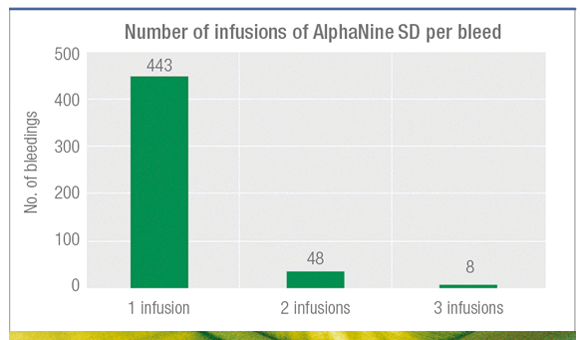
89% of bleedings resolved with 1 infusion1
Twenty-one adverse events were reported in 8 patients, none of which was considered related to the study medication. After 12 month follow-up, no evidence of inhibitor formation in treated subjects. No clinical or lab evidence of allergic reactions or thrombotic events throughout the entire study period.1
AlphaNine SD infused pre- and post-operatively is an effective treatment for hemophilia B patients undergoing major surgery2
n = 29 major surgeries (90% orthopedic and 10% abdominal)2
Study of 20 hemophilia B patients undergoing major surgery treated with AlphaNine SD – a single infusion retrospective analysis.2
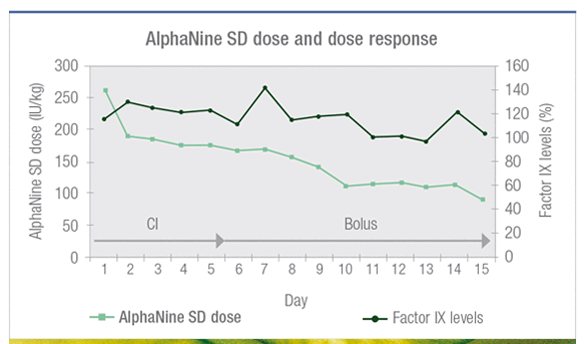
AlphaNine SD response was monitored throughout the hospital stay and the dose was adjusted accordingly with a target peak level at 100% (with continuous infusion or bolus).2
Indication
AlphaNine® SD (coagulation factor IX [human]) is indicated for the prevention and control of bleeding in patients with Factor IX deficiency due to hemophilia B.
AlphaNine SD is made from human plasma. Plasma products carry a risk of transmitting infectious agents, including viruses, and theoretically, the Creutzfeldt-Jakob disease (CJD) agent, despite steps designed to reduce this risk.
Incidences of thrombosis or disseminated intravascular coagulation (DIC) have been reported following administration of Factor IX Complex concentrates which contain high amounts of Factor II, VII, and X. AlphaNine SD contains low, nontherapeutic levels of Factor II, VII, and X.
Following administration in surgery patients and individuals with known liver disease, the physician should closely observe the patient for signs and symptoms of potential disseminated intravascular coagulation.
Allergic type hypersensitivity reactions, including anaphylaxis, have been reported for all factor IX products. The administration of plasma preparations may cause allergic reactions, mild chills, nausea or stinging at the infusion site.
Nephrotic syndrome has been reported following attempted immune tolerance induction with factor IX products in hemophilia B patients with factor IX inhibitors and a history of severe allergic reactions to Factor IX.
In order to minimize the possibility of thrombogenic complications, dosing guidelines should be strictly followed.
AlphaNine SD should not be administered at a rate exceeding 10 mL/ minute. Rapid administration may result in vasomotor reactions.
Please see full Prescribing Information for AlphaNine SD.
References
- Lissitchkov T, Matysiak M, Zavilska K, Laguna P, Gercheva L, Antonov A, Cabrera N, Aznar JA, Woodward MK, Páez A. A clinical study assessing the pharmacokinetics, efficacy and safety of AlphaNine(®) , a high-purity factor IX concentrate, in patients with severe haemophilia B. Haemophilia. 2011 Jul;17(4):590-6.
- Quon DV, Logan L. Safety and efficacy of plasma-derived coagulation factor IX concentrate (AlphaNine® SD) in patients with haemophilia B undergoing surgical intervention: a single institution retrospective analysis. Haemophilia. 2011 Jan;17(1):e196-201.


