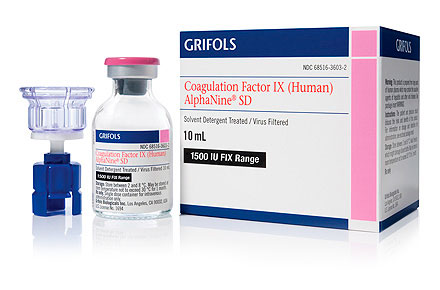
How Supplied
For your patients with hemophilia B
Three convenient vial sizes with 10 mL diluent
| Potency | Diluent size | NDC numbers |
|---|---|---|
| 500 IU FIX range | 10 mL | 68516-3607-2 68516-3610-2 |
| 1000 IU FIX range | 10 mL | 68516-3608-2 68516-3611-2 |
| 1500 IU FIX range | 10 mL | 68516-3609-2 68516-3612-2 |
| Potency | 500 IU FIX range |
| Diluent size | 10 mL |
| NDC numbers | 68516-3607-2 68516-3610-2 |
| Potency | 1000 IU FIX range |
| Diluent size | 10 mL |
| NDC numbers | 68516-3608-2 68516-3611-2 |
| Potency | 1500 IU FIX range |
| Diluent size | 10 mL |
| NDC numbers | 68516-3609-2 68516-3612-2 |
- A Mix2Vial® included in every carton for easy reconstitution
- Room temperature storage for up to 1 month
Indication
AlphaNine® SD (coagulation factor IX [human]) is indicated for the prevention and control of bleeding in patients with Factor IX deficiency due to hemophilia B.
AlphaNine SD is made from human plasma. Plasma products carry a risk of transmitting infectious agents, including viruses, and theoretically, the Creutzfeldt-Jakob disease (CJD) agent, despite steps designed to reduce this risk.
Incidences of thrombosis or disseminated intravascular coagulation (DIC) have been reported following administration of Factor IX Complex concentrates which contain high amounts of Factor II, VII, and X. AlphaNine SD contains low, nontherapeutic levels of Factor II, VII, and X.
Following administration in surgery patients and individuals with known liver disease, the physician should closely observe the patient for signs and symptoms of potential disseminated intravascular coagulation.
Allergic type hypersensitivity reactions, including anaphylaxis, have been reported for all factor IX products. The administration of plasma preparations may cause allergic reactions, mild chills, nausea or stinging at the infusion site.
Nephrotic syndrome has been reported following attempted immune tolerance induction with factor IX products in hemophilia B patients with factor IX inhibitors and a history of severe allergic reactions to Factor IX.
In order to minimize the possibility of thrombogenic complications, dosing guidelines should be strictly followed.
AlphaNine SD should not be administered at a rate exceeding 10 mL/ minute. Rapid administration may result in vasomotor reactions.
Please see full Prescribing Information for AlphaNine SD.
